Abstract
Introduction: Lymphoplasmacytic lymphoma/ Waldenström Macroglobulinemia (LPL/ WM) is a rare B-cell malignancy and constitutes 1%-2% of non-Hodgkin lymphoma. Because of the low incidence of this disease, there is wide heterogeneity in the approaches and regimens for the symptomatic patients. In recent years, BTK inhibitors have performed good efficacy for WM patients. The response rate of single-agent for newly diagnosed or relapsed/refractory patients is up to 80-90%. However, the deep remission rate of BTK inhibitor is low, and the rate of very good partial response (VGPR) is only 20-30%. Ixazomib, a proteasome inhibitor that can inhibit NF-kB signaling pathway activation is also widely used in WM patients. Previous clinical studies have shown that the response rate of ixazomib combined with rituximab and dexamethasone in newly diagnosed WM is up to 96%, and the VGPR rate is 15%. How to further improve the depth of remission is the key to enhance the efficacy of WM patients. Herein, we performed a phase 2 investigator-initiated clinical trial (NCT04463953) to evaluate the efficacy and safety of the combination of zanubrutinib, ixazomib, and dexamethasone (ZID) in newly diagnosed symptomatic WM patients.
Methods: Patients received ID (Ixazomib 4 mg orally days 1, 8, 15; dexamethasone 20 mg orally days 1,2, 8,9, 15,16) induction therapy in 28-day cycle for up to six cycles. After induction therapy, maintenance therapy was conducted every three months. Zanubrutinib was administered 160 mg twice a day in the whole treatment period and discontinued in the last month, ending with ID regimen. Treatment duration will be maximum twenty-four months. The primary endpoint was the deep remission (≥VGPR) rate after six courses of induction therapy.
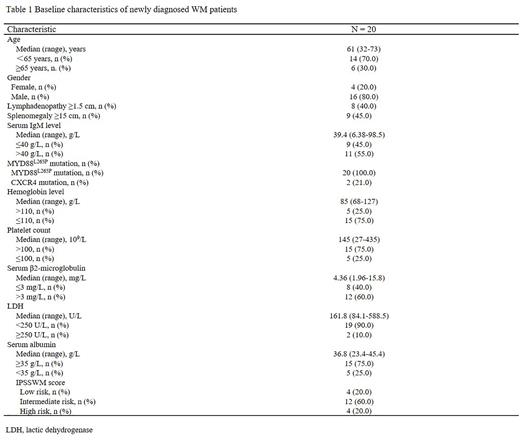
Results: From Jun 2020 to Feb 2022, twenty patients were enrolled in this study. The clinical characteristics were described in Table 1. One patient did not complete all six cycles induction study, and therefore the efficacy analysis was performed on nineteen patients. After induction therapy, eight patients achieved VGPR, ten patients achieved partial remission (PR), and one patient had minor response (MR). The overall, major, and very good partial response rates were 100%, 94.7%, and 42.1%, respectively. Median onset-to-treatment (MR or better) time and optimum reaction time was 1.1 months (range: 0.7-3.1) and 3.7 months (range: 0.9-17.1), respectively. After treatment, all patients recovered normal hemoglobin levels and two (10.5%) patients achieved MRD negative marrow response by flow cytometry. Spleen volume and lymph node size returned to normal in six (85.7%) and two (25.0%) patients. The median follow-up time was 12.3 months (range: 5.4-23.4), all patients are alive. One patient experienced disease progression after cessation of treatment for 2.5 months. Before progression, the patient had received a total of twenty-one months treatment and achieved a PR response. Two patients experienced an IgM rebound after cessation of treatment for one and four months, respectively, but they were both asymptomatic and had no indication for secondary treatment. Prior to that, they received treatment for 19 and 21 months, respectively, and both achieved VGPR responses. Insomnia was the most frequently reported grade 1-2 adverse event (n=5; 25%). Serious adverse reactions (grade ≥3) were observed in two patients (10%) including rash and neutropenia in each.
Conclusion: The zanubrutinib plus ixazomib and dexamethasone regimen has a high rate of deep remission in newly diagnosed symptomatic WM patients with limited adverse events. The study is ongoing and further results will be continuously released.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal